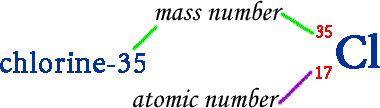|
Isotopes are two or more atoms of the same element with the same number of protons but a different number of neutrons. The existence of isotopes proves that part of Dalton's atomic theory is incorrect. Dalton wrote that atoms of the same element have the same physical and chemical properties. Although isotopes have the same chemical properties, they do not have the same physical properties. Because the number of neutrons is different, the mass number is different. Isotopes are written in two different ways. They can be written using their symbol with the mass number (to the upper left) and atomic number (to the lower left) or the isotope name is written with a dash and the mass number.
For example: Two naturally occurring isotopes of chlorine are chlorine-35 & chlorine-37. Thirty-five and thirty-seven are the mass numbers for the two isotopes. Both isotopes have the same number of protons (17).
The number of neutrons is determined by subtracting the number of protons from the mass number; (35-17 = 18 & 35-17 = 20 respectively). |