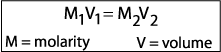| Stock solutions are often available in a chemistry laboratory. Often times, however, chemists need to dilute a stock solution to produce a less concentrated solution. In order to make a more dilute solution, more solvent is added. To determine the exact amount of stock solution to dilute, the following formula is used: |
|
For example: How would you prepare 500 mL of a 0.1 M saline solution from a stock 2.5 M solution?
Plug in your variables and solve for x. Explain the process for making the dilution: |