| 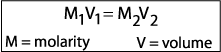
For
example: How would you prepare 500 mL of a 0.1 M saline solution
from a stock 2.5 M solution?
Plug in your variables and solve for x.
(0.1 M) ( 500 mL) = (2.5 M) (x)
x = 20 mL
Explain the process for making the dilution:
Take 20 mL of stock solution and dilute it to make a 500 mL solution.
Note: 20 mL was what you determined that you needed from your stock solution
and 500 mL was the final volume needed. You should always accompany your
problem with an explanation on how to perform the dilution.
|

