Gas
Law Formulas
Ptotal
= P1 + P2 + P3
...
Dalton’s
Law of Partial Pressure
X1 = n1/ntotal = P1/Ptotal
Mole
Fraction
P1V1
= P2V2
Boyle’s
Law
V1 /
T1
= V2 / T2
Charles’
Law
V1 /
n1
= V2 / n2
Avogadro's
Law
P1 /
T1
= P2 / T2
Gay-Lussac’s
Law
P1V1 / T1 = P2V2 / T2
Combined
Gas Law
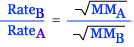
Graham's
Law
PV
= nRT
R = 8.3145 L kPa/mol K or
R= 0.08206 L atm/mol K
R = 8.3145 L kPa/mol K or
R= 0.08206 L atm/mol K
Ideal
Gas Law
(mm) P
= dRT
mm = molar mass
d = density
R= 0.08206 L atm/mol K
mm = molar mass
d = density
R= 0.08206 L atm/mol K
Gas
Density/Molar Mass
vrms
=
√(3RT
/ M)
M = molar mass in kg / mol
R = 8.3145 J/mol K
M = molar mass in kg / mol
R = 8.3145 J/mol K
Root
Mean Square Velocity
[Pobs + a(n/V)2] x (V – nb) = nRT
van
der Waals Equation
Standard
Atmospheric Pressure:
1 atm = 760 torr = 760 mm Hg = 101.3 kPa = 14.7 psi
1 atm = 760 torr = 760 mm Hg = 101.3 kPa = 14.7 psi